|
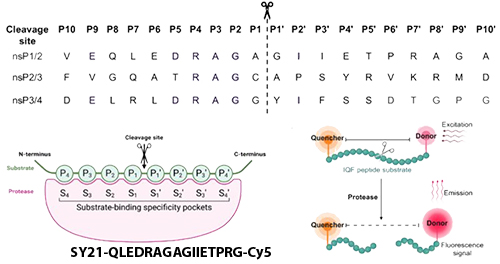
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus causing debilitating fever, joint pain, and neurological complications. Lacking approved antivirals, researchers have identified the virus’s nonstructural protein 2 (nsP2) protease as a vital target-this cysteine protease orchestrates the cleavage of the viral polyprotein and is essential for productive infection.
A critical insight from the 2024 study in PNAS was the creation of an internally quenched peptide based on the CHIKV nsP2-mediated cleavage junction-QLEDRAGA/GIIETPRG. This substrate underpinned a high‑throughput FRET-based enzymatic assay for screening covalent inhibitors, facilitating the discovery of RA‑0002034, a nanomolar inhibitor that covalently modifies the protease’s catalytic cysteine and demonstrates potent antiviral effects.
What Makes This Sequence Special?
Biological Significance
- Exact mimic of the nsP2 1/2 cleavage site—ensuring physiological relevance.
- Enables direct monitoring of native protease activity via fluorescence.
Assay Performance
- When uncleaved, quencher suppresses fluorescence.
- Upon protease cleavage, Cy5 fluorescence is unmasked, yielding a clear, quantifiable signal ideal for high-throughput assays.
Therapeutic Discovery Platform
- Used to screen over 6,000 covalent fragments, leading to a powerful inhibitor with IC₅₀ in the low nanomolar range.
- Supported kinetic characterization (k_inact/K_I), LC‑MS confirmation, and antiviral validation in cell models.
The QSY21-QLEDRAGAGIIETPRG-Cy5 peptide is a purpose-built, scientifically validated reagent for profiling CHIKV nsP2 protease activity. As demonstrated by its pivotal role in identifying a potent covalent inhibitor, this substrate is a powerful tool for early-stage antiviral drug discovery, especially against CHIKV and potentially other alphaviruses. Use it in FRET-based assay platforms to accelerate your protease inhibitor screening projects.
References:
1. Ghoshal, A., Tse, E.G., Hossain, M.A. et al. A covalent chemical probe for Chikungunya nsP2 cysteine protease with antialphaviral activity and proteome-wide selectivity. Sci Rep 15, 7264 (2025), https://doi.org/10.1038/s41598-025-91673-x.
2. E.M. Merten, J.D. Sears, T.M. Leisner, P.B. Hardy, A. Ghoshal, M.A. Hossain, K.H. Asressu, P.J. Brown, E.G. Tse, M.A. Stashko, K. Li, J.L. Norris-Drouin, L.E. Herring, A.L. Mordant, T.S. Webb, C.A. Mills, N.K. Barker, Z.J. Streblow, S. Perveen, C.H. Arrowsmith, R.M. Couñago, J.J. Arnold, C.E. Cameron, D.N. Streblow, N.J. Moorman, M.T. Heise, T.M. Willson, K.I. Popov, & K.H. Pearce, Identification of a cell-active chikungunya virus nsP2 protease inhibitor using a covalent fragment-based screening approach, Proc. Natl. Acad. Sci. U.S.A. 121 (42) e2409166121, https://doi.org/10.1073/pnas.2409166121 (2024).
|
 https://www.lifetein.com
100 Randolph Road, Suite 2D,
Somerset
USA
New Jersey
08873
https://www.lifetein.com
100 Randolph Road, Suite 2D,
Somerset
USA
New Jersey
08873
 https://www.lifetein.com
100 Randolph Road, Suite 2D,
Somerset
USA
New Jersey
08873
https://www.lifetein.com
100 Randolph Road, Suite 2D,
Somerset
USA
New Jersey
08873