LifeTein offers stapled peptide synthesis and special amino acids and modifications, including Fluoromethylketone (FMK), Glycosylation, chloromethylketone (CMK), N-methyl amino acids, unnatural amino acids, acetyl-lysine, beta-alanine, aminobenzoic acid, amidation, acetylation, Abu, citrulline, Acm, dimethyl-lysine, hydroxy-proline (Hyp), methyl-lysine, mercaptopropionic acid, nitro-tyrosine, norleucine (Nle), pyro-glutamic acid (Pyr), carbobenzoxyl (Z), succinic acid, and sulfurylation.
LifeTein offers stable isotope labeled peptides with amino acids enriched in 13C, and 15N. These 13C/15N peptides are similar to their native peptides in terms of chemical, physical properties, and biological activities. These peptides are used to study protein interactions and post-translation modifications such as ubiquitination and phosphorylation.
Peptide stapling is a strategy for constraining short peptides in an alpha-helical conformation. LifeTein's stapling is carried out by covalently linking the side-chains of two amino acids, thereby forming a peptide macrocycle. The stapled peptides can be used for intracellular drug targets because stapling can increase the target affinity and proteolytic resistance. The hydrocarbon-stapling is achieved by ring-closing olefin metathesis between two functionalized non-natural building blocks, either R8-S5 or S5-S5. S5 stands for S-2-(4′-pentenyl) alanine. R8 stands for R-2-(7′-octenyl) alanine.
Hydrocarbon stapled α-helical peptides are capable of targeting and interfering with intracellular protein-protein interactions. These peptides contain a synthetic brace, introduced across one face of an α-helix. The structure can increase α-helical content and protease resistance, enhance target binding affinity, and promote cell membrane penetration.Three types of stapled peptides are used with optimized combinations of non-natural building blocks such as (R)-N-Fmoc-2-(7'-octenyl) alanine and (S)-N-Fmoc-2-(4'-pentenyl) alanine. For example, the stitched peptide can be generated by the introduction of S5 at the i position, B5 at the i + 4 position, and S8 at the i + 11 position.


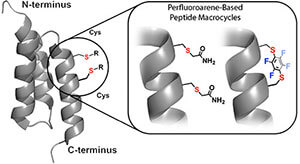
Perfluoroarene-Based peptide macrocycles contain rigid perfluoroaromatic staples. This method is selective towards cysteines and provides staples featuring lipophilic perfluorinated moieties.
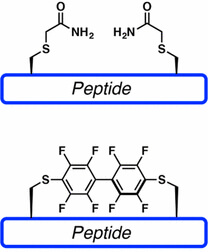
Perfluoroarene-based peptide macrocycles: This stapling modification performed on a peptide sequence showed enhancement in binding, cell permeability, and proteolytic stability properties, as
compared to the unstapled analog.
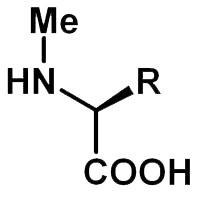
N-methyl α-amino acids are important building elements of many naturally occurring antibiotics. N-methyl amino acids are known to increase pharmacokinetically useful parameters such as membrane permeability, proteolytic stability, and conformational rigidity. Most peptides can be hydrolyzed easily by digestive enzymes, or are poorly absorbed through the intestine, resulting in poor oral availability. The substitution of N-methyl amino acids can increase peptidase stability and enhance intestinal permeability.
LifeTein's methods for the preparation of N-methyl amino acids and their N-protected derivatives include the direct alkylation of Na-Boc or Cbz protected amino acids, the reductive methylation of N-benzyl amino acids followed by hydrogenolysis, or the reduction of N-Fmoc-oxazolidinones or methylol derivatives with triethylsilane in TFA. However, amino acids with reactive side chains could be difficult to handle. Therefore, different strategies of side chain protection or assembly should be adopted for each amino acid to guarantee successful synthesis.
The stapled peptides are protein fragments chemically locked into an α-helical shape. Helix stabilization by cross-linking had been shown previously to increase the helicity and potency of α-helical peptides dramatically. The stapled peptides can selectively target only one of a closely related family of proteins. They are a promising class of alpha-helix mimetic inhibitors designed to be resistant to degradation, to penetrate cell membranes, and to bind tightly to disease target proteins.
Unusual amino acids incorporated into a peptide can:
Please click here to get a peptide synthesis service quote now!