|
BP100 (KKLFKKILKYL‑NH₂) is a cationic antimicrobial peptide originally designed to target Gram‑negative bacterial membranes through a carpet‑like mechanism—transitioning to α‑helical structure upon binding, inserting into membranes, and promoting permeabilization. It functions preferentially against anionic membranes while maintaining low toxicity to plant or mammalian cells.
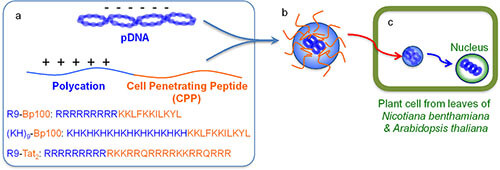
RRRRRRRRRKKLFKKILKYL-NH2. R9-BP100 is a cell-penetrating peptide that can be used as a delivery tool for rapid and efficient gene delivery into plant cells. R9-BP100 is made up of nine tandem polyarginine R9 (RRRRRRRRR) and BP100 (KKLFKKILKYL). R9 is a derivative of TAT where all amino acids are substituted for arginine. R9 was known for its effectiveness in both plant and mammalian systems as well as its relatively well-characterized mechanism of action in mammalian cells.
BP100 was originally designed and optimized as an antimicrobial peptide to protect against plant pathogens. R9 appeared to be the most effective of the tested CPPs, with TAT being 0.77 times as effective as R9, and BP100 or GFP11 alone showing no statistically significant signal. BP100 inhibits twitching motility of Xylella fastidiosa—limiting systemic spread in plants without killing cells outright. It has a concerted membrane-binding α-helical mechanism, causing carpet-like disruption in anionic bacterial membranes. Beyond antimicrobial uses, BP100 analogs like BP100‑W have anti‑inflammatory and antibiofilm properties, suggesting possible synergy with delivery or defense‑eliciting applications.
(doi: https://doi.org/10.1101/2022.05.03.490515). Image reference: Biomacromolecules 2013, 14, 1, 10-16.
|