|
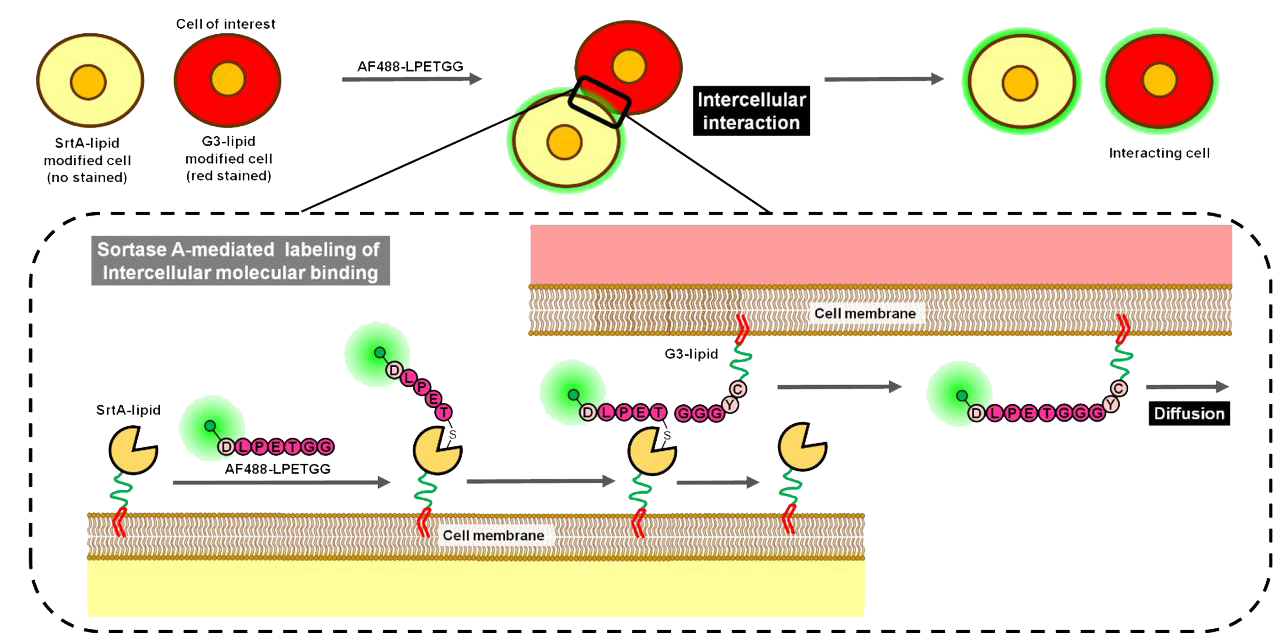
The peptide sequence Cys-LPETGS is a synthetic construct that combines a cysteine residue at the N-terminus with the LPETG motif, a well-characterized recognition sequence for the enzyme sortase A (SrtA). The LPETG motif is crucial for SrtA-mediated transpeptidation reactions, where SrtA cleaves between the threonine and glycine residues, facilitating the covalent attachment of proteins to the cell wall in Gram-positive bacteria. The addition of an N-terminal cysteine introduces a reactive thiol group, enabling site-specific conjugation through maleimide chemistry, which forms stable thioether bonds with thiol-containing molecules.
Applications:
The dual functionality of Cys-LPETGS makes it a versatile tool in biochemical and biomedical research. The N-terminal cysteine enables conjugation with maleimide-activated fluorescent dyes, such as Alexa Fluor 488, Cy5, and Cy7, facilitating applications in imaging and flow cytometry. Additionally, the peptide can be conjugated to lipids like DSPE-PEG2000, enabling the incorporation of proteins or peptides into liposomal membranes for targeted drug delivery systems. The LPETG motif permits enzymatic ligation to proteins or peptides bearing N-terminal oligoglycine sequences via SrtA, allowing for the construction of complex, site-specific bioconjugates. This combination of chemical and enzymatic conjugation strategies expands the utility of Cys-LPETGS in the development of multifunctional biomolecules.
Biological Importance:
The LPETG motif within Cys-LPETGS is recognized by SrtA, which plays a pivotal role in anchoring surface proteins to the cell wall in Gram-positive bacteria, a process essential for bacterial virulence and adherence. By mimicking this natural substrate, Cys-LPETGS serves as a valuable tool for studying SrtA-mediated processes and for engineering proteins with defined orientations and functionalities. The inclusion of the N-terminal cysteine allows for precise chemical modifications, facilitating the development of targeted therapeutics, imaging agents, and vaccine candidates. The ability to combine enzymatic specificity with chemical versatility makes Cys-LPETGS a powerful component in the design of advanced biomolecular constructs.
An illustrative example of utilizing the LPETG motif for fluorescent labeling is presented in a study published in Nature Communications. In this research, scientists employed an evolved sortase A (eSrtA) enzyme to catalyze the conjugation of Alexa Fluor 750-labeled LPETG peptides onto pentaglycine-modified polyurethane catheters implanted in mice. This enzymatic reaction facilitated real-time, in vivo fluorescent imaging of the catheter surfaces.
The study demonstrated that the fluorescent signal from the LPETG-tagged probes could be effectively removed by administering eSrtA along with a triglycine peptide, showcasing the reversible nature of this labeling technique. This approach underscores the potential of LPETG-mediated conjugation for dynamic and controllable surface modifications in biomedical applications.
Reference: Ham, H., Qu, Z., Haller, C. et al. In situ regeneration of bioactive coatings enabled by an evolved Staphylococcus aureus sortase A. Nat Commun 7, 11140 (2016). https://doi.org/10.1038/ncomms11140
|