The LifeTein Protein Analysis Tool is a free bioinformatics protein sequence analysis software program. It incorporates exclusive and powerful bioinformatics algorithms to design and analyze peptide antigens to ensure that each project results in a custom highly specific antibody with a high-titer.
The Protein Analysis Tool is a web-based software program that calculates the chemical formula of a peptide, as well as its molecular weight, net charge at neutral pH, hydroelectric point, hydrophilicity and hydrophobicity, and extinction coefficient. The program can accurately predict important structural characteristics of the input protein and peptide sequences such as hydrophobic or hydrophilic regions, secondary structure, transmembrane regions, flexible regions, and the surface probability.
Applications
Peptides are complex biological molecules with unique physical and chemical properties, which result directly from their amino acid composition. Peptides can be designed either de novo, or can be based on the sequences of native proteins, depending on the final application. To maximize the probability that antibodies directed against synthesized peptides will recognize native proteins in target assays, a suitable peptide sequence must be selected for antigen design. Below, we outline a number of important principles that should be considered during the peptide design process. The technical support department at LifeTein can also assist you with peptide design.
Generally, a good peptide antigen is derived from a sequence located on the surface of the native protein. An optimal sequence has a flexible structure (usually a loop) with a unique sequence, is easy to synthesize, and possesses no sited that are modified post-translationally.
Homology Considerations:
When designing a peptide antigen, two basic strategies should be considered:
Generally, most optimal antigenic epitopes are flexible, hydrophilic, located on the surface of the protein. This is because hydrophilic protein regions are commonly located on the surface of proteins, whereas hydrophobic regions tend to be inside the protein in most natural environments. In addition, antibodies can bind only to epitopes that are located on a protein surface, and generally bind with higher affinity when the epitopes are flexible and readily move into most accessible positions.
Continuous Epitopes, Discontinuous Epitopes:
X-ray crystallography can be used to determine the structure of proteins, which helps scientists visualize macromolecular protein complexes, predict the effect of specific amino acid mutations, and also design targeted therapeutic agents. However, proteins are generally more flexible that their three-dimensional structures would suggest. Intracellular proteins fold and unfold continuously. Most antibodies target a continuous amino acid sequence, or continuous epitopes. Antibodies bind to these regions with high affinity if those sequences are located toward the surface of the protein. However, antibodies can also be generated against discontinuous epitopes in instances where the epitopes are against a fold within a peptide sequence, or in a location where two distinct peptide chains meet. However, the peptide used for immunization must have a similar secondary structure to the epitope for this to be successful.
If the final research application focuses on specific protein domains, such as the N- and C-termini, or a specifically altered protein state, such as phosphorylation, the peptide antigens and the resulting antibodies are relatively easy to use. However, the conformation of target proteins might prevent the antibody from accessing the target epitopes. For example, a particular sequence could become inaccessible if it is hidden inside of a folded protein. Therefore, this is an important consideration during peptide design for antibody recognition purposes.
Targeting the N-terminus or C-terminus:
To reduce the chances of designing an epitope buried within a protein, we generally recommend that antibodies are designed against the C- or N-terminal regions of proteins, which are often exposed. However, it is important to note that transmembrane domains and the C-terminal sequences of many membrane proteins are commonly too hydrophobic to be used as antigens.
Sequence Length:
Generally, we recommend that peptide sequences of 8-20 amino acids should be used for antibody preparation. If the peptide is too short, it is unlikely to be specific enough to allow the resulting antibodies to recognize the target native protein with sufficient affinity. Conversely, sequences longer than 20 amino acids might lose their specificity and induce secondary reactions.
Peptide Purity: A peptide purity of 75% is generally sufficient for antibody generation and testing. However, 95% purity is recommended for studying biological activity.
Peptide Solubility: The amino acid composition of a peptide strongly influences its solubility. Ideally, the hydrophobic amino acid content should be kept below 50%, and there should be a minimum of one charged residue for every five amino acids.
Amino acid classifications
Hydrophobic (non-polar) amino acids: Ala, Ile, Leu, Met, Phe, Trp, Val
Uncharged (polar) amino acids: Asn, Cys, Gly, Gln, Pro, Ser, Thr, Tyr
Acidic (polar) amino acids: Asp, Glu
Basic (polar) amino acids: His, Lys, Arg
Peptides with a large number of hydrophobic amino acids have limited solubility in aqueous solutions; some are insoluble completely. These peptides are commonly difficult to use experimentally and can be difficult to purify. At physiological pH, acidic (Asp, Glu) and basic (Lys, Arg) amino acids all have charged side chains. Some hydrophobic amino acids located in nonessential positions could be substituted for conservative amino acids such as alanine or glycine, removed altogether, or replaced with analogs. In addition, a single conservative substitution or the addition of some polar residues to the N- or C-terminus of a peptide could improve its solubility.
There are a number of simple characteristics that can be used to predict solubility:
Although coupling strategies often vary according to the sequence of the peptide, the carrier proteins should be linked to the peptide via C- or N-terminal residues. If there are no internal cysteine residues, then a cysteine should be added to the sequence. Generally, we recommend that the carrier protein should be conjugated to the N-terminus of the peptide. Peptides of interest are conjugated to carrier proteins that contain many epitopes to stimulate T-helper cells because most peptides alone are too small to elicit an immune response large enough to lead to antibody generation. The T-helper cells induce the B-cell response that generates the antibodies. The immune system recognized the peptide-carrier protein complex as though it was a whole protein. Therefore, some antibodies target the linker region and the carrier protein itself. Nevertheless, these non-specific antibodies can be removed during purification. There are several common carrier proteins that are used during antibody production.
All modifications carrying thiol-reactive functional groups can be used. There are three commonly used reactive groups:
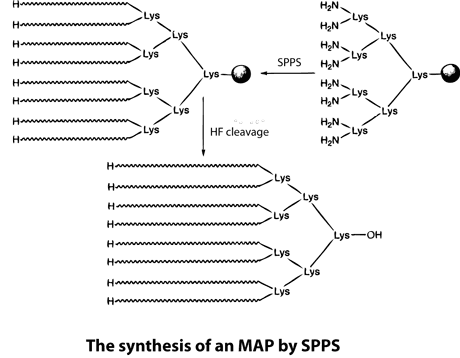
Multiple antigenic peptides (MAPs) are artificially branched peptides. Lysine residues are used as the scaffolding core to form up to eight branches that have the same or different peptide sequences. MAPs have been used for antibody production in immunological studies for a long time. Although some peptides render a lower immunological response, new structures, such as concentrated branched peptides, can increase the immunological response dramatically. Many antigenic peptides can be synthesized using standard SPPS. During this process, Boc-Lys(Boc)-OH is anchored to a resin, the peptides are treated with TFA, and cycles of deprotection and coupling are then performed sequentially. Peptides to be used in immunological studies are then synthesized as each of the eight branches. However, the nature of branched proteins is unknown, and so MAP synthesis can be challenging. For example, the space between the eight branches can cause the peptides to aggregate on the resin, which results in low coupling yields and/or deletions.
. To overcome this problem, LifeTein uses PeptideSyn technology for chemical ligation. This allows the desired peptide dendrimer to be formed at higher yields than could be achieved using traditional methods. The branched structures result in a higher molecular weight of the protein, which aids immunogenicicty.
Peptide Modifications: An important advantage of synthetic peptides is that they can be synthesized into exact conformations or according to the specific characteristics that are required for the end application. Peptides in general and certain amino acids contain distinct moieties that can be modified: an N-terminal amino group, a C-terminal carboxyl group, the alpha-amino group of lysines, the hydroxyl group of serine, threonine, and tyrosine residues, arginine residues containing guanidine groups, and the thiol group of cysteine. A. N-terminal amino group B. C-terminal carboxyl group C. alpha-Amino group on lysine D. Hydroxyl group on serine, threonine, and tyrosine E. Guanidine group on arginine F. Thiol group on cysteine
Although the list shown above includes the most common modifications, it is by no means complete. Some modifications occur post-translationally in vivo, whereas others take the form of the substitution of natural amino acids for non-natural variants. In addition, specific tags or proteins can be cross-linked to the moieties described above. Because of the C-to-N direction of peptide synthesis, we recommended that tags and dyes be conjugated to the N-terminus of the peptide so that only the full-length sequences are labeled.
|
|
||
Reference:
References using synthetic peptides and antibodies from LifeTein. See search results page from the Google Scholar.
Polyclonal rabbit antibodies to Ll-Bhp-1 were commercially produced using recombinant Ll-Bhp-1 and purified by protein A affinity chromatography (LifeTein, Somerset, NJ, USA).
LifeTein produced a series of snake venom antibodies. Venom species used for rabbit IgG production: Tiger, Brown, Black, Death adder, and Taipan. The snake venoms were used for immunization of rabbits to generate polyclonal antibodies.
Protein A purified rabbit IgG specific for each venom species (Table 1) were purchased from a commercial supplier (Lifetein, Somerset, NJ, USA).
LifeTein designed and synthesized a series of peptides: CENH3, MIS12 and NDC80. The peptides were used for immunization of rabbits to generate polyclonal antibodies.
... The peptide synthesis, immunization, and antibody purification were performed by LifeTein...
LifeTein generated Anti-gM and anti-gN antibodies for Vaccine Analytical Research Development and Vaccine Process Development Merck & Co., Inc., Kenilworth, NJ, USA. The rabbit polyclonal anti-gM and anti-gN antibodies were elicited by vaccinating animals with synthesized peptide sequences of gM (1-13aa and 345-372aa) and gN (61-101aa). Animal immunization and sera collection were performed at Lifetein, LLC (Hillsborough, NJ, USA). The sera were affinity-purified by its corresponding peptides.
LifeTein helped designed and synthesized a series of phosphorylated peptides. Then the peptides were used for phospho-specific antibody productions. The phospo-specific antibodies by LifeTein were confirmed to react with the epidermal growth factor receptor (EGFR). The Hung's lab showed that AGO2-Y393 phosphorylation mediates EGFR-enhanced cell survival and invasiveness under hypoxia. These findings suggest that modulation of miRNA biogenesis is important for stress response in tumour cells.
... The following peptides were chemically synthesized for antibody production in mice (Lifetein), Elisa verification (LifeteinConc.) and peptide competition assay in immunohistochemistry (IHC)... Supplementary information
George R. Tiller, Potential Roles of Peroxidases in Caenorhabditis Elegans Innate Immunity. 2014
Custom peptide antibodies were provided by LifeTein to study SKPO-1. The SKPO-1 peroxidase domain peptide was synthesized by LifeTein. The polyclonal antibodies were raised in rabbits and then affinity purified to accomplish high specifications and concentrations. The antibodies were successfully used to localize the SKPO-1 protein in hypodermis. It was found that the peroxidase SKPO-1 contributes to the host immune response during infection.
... With respect to having a pAb raised for IHC, I designed a synthetic SKPO-1 peroxidase domain peptide to be chemically synthesized and have a pAb raised in rabbits against said peptide by Lifetein®. This endeavor was successful and is covered in Chapter 3...
LifeTein helped synthesized a series of phosphorylated peptides. Then the peptides were used for phospho-specific p-METTL3 (S43) antibody productions. Sun et al. demonstrate that activation of the ERK pathway promotes m6A methylation by phosphorylation of METTL3 and WTAP.
LifeTein made the affinity-purified rabbit polyclonal anti-SclA antibody. The purified streptococcal collagen-like protein A (SclA) antibodies demonstrated that a mutation in Group A Streptococcus carrier strains increase adherence and decrease virulence.
LifeTein designed and synthesized a series of phosphorylated peptides. ELISA and dot blot analysis were performed for the verification of antibody specificity.
... Antibodies to the Thr345, Ser416, and Ser497 phosphorylation sites of XPO5 were generated in collaboration with Lifetein LLC... Supplementary information