Most of the potential therapeutic peptides have low solubility, chemical instability or low stability against protease. So it is essential to modify and optimize the peptides to improve the peptide bioavailability.
One novel approach is to create a self-assembly, highly ordered, and stable nanostructure. For instance, many peptide hormones including glucagon are stored in the form of β-sheet rich amyloid-like fibrils via a hydrogen bond network in the secretory cell.
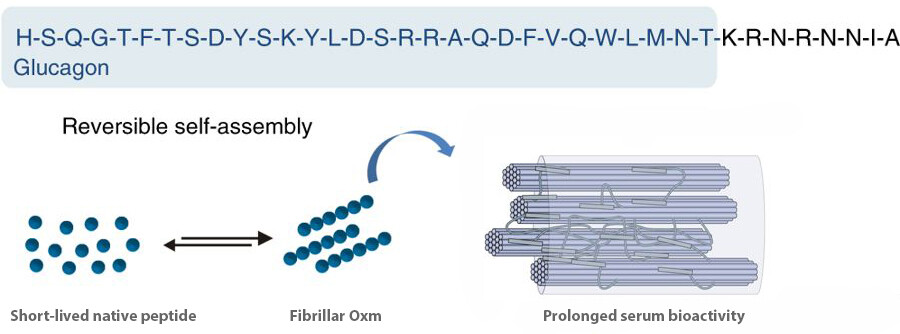
The oxyntomodulin is a peptide with a potential to treat obesity and diabetes. It is a 37-amino-acid proglucagon-derived peptide hormone with sequence homology to both glucagon and glucagon-like peptide-1 (GLP-1). The oxyntomodulin peptide self-assembles into a stable nanofibril formulation and later on releases an active peptide.
Here is the sequence of human oxyntomodulin: His-Ser-Gln-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Lys-Tyr-Leu-Asp-Ser-Arg-Arg-Ala-Gln-Asp-Phe-Val-Gln-Trp-Leu-Met-Asn-Thr-Lys-Arg-Asn-Arg-Asn-Asn-Ile-Ala, HSQGTFTSDY SKYLDSRRAQ DFVQWLMNTK RNRNNIA.
Oxyntomodulin self-assembles into fibrillar nanostructures
- The peptide concentration was 10 mg/mL in water at an incubation pH of between 7.0 and 7.3 and low ionic strength (0.09% saline).
- The solution was incubated at 37 °C and agitated by orbital shaking.
- After incubation for five days, the solution turned turbid due to the formation of a suspension of aggregates. The conversion yield of the self-assembly of Oxyntomodulin into fibrillar nanostructures was estimated to be 99% under these conditions.
- The nanofibrils were next used to seed a solution of free peptide at 10 mg/mL in water. 5. The solution was incubated without agitation for one week and then diluted to 1 mg/mL in 0.09% saline for another 2–9 days of incubation at 37 °C and then nine days at room temperature.
- Free peptides were mainly in an α-helical conformation. The fibrillar Oxm showed the majority of β-sheet and some α-helical content.
Peptide nanofibrils dissociate to release intact peptide
- 1 mg/mL nanofibrils were incubated in water. 37% of the peptide was released after four hours of incubation.
- In aqueous HCl, a 77% release was observed after only four hours. The peptide remained chemically intact after discharge from the nanofibrils
Benefits:
- The released peptide is active and nontoxic in vitro.
- The nanofibrils prolong peptide serum bioactivity in vivo. And there is no need to engineer or modify the original peptide. Other peptide hormones including glucagon, GLP-1, exendin-4, calcitonin, and gastric inhibitory peptide, are known to self-assemble. The self-assembly method may be used for the clinical application of reversibly self-assembling nanofibrils.
Reference:
Controlling the bioactivity of a peptide hormone in vivo by reversible self-assembly. Nature Communications, volume 8, Article number: 1026 (2017)