Peptides can be modified at the N terminus, in the middle, or at the C terminus. In general, some standard peptide moieties must be accessible in order to be modified: an N-terminal amino group, the amino group of Lys, the thiol group of Cys, the hydroxyl groups of Ser, Thr, and Tyr, the C=NH group of Arg, and the C-terminal carboxyl group.
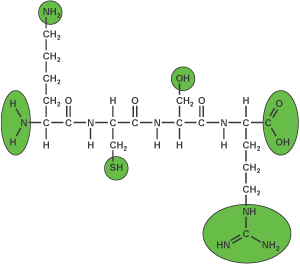
Chemically synthesized peptides have free N- and C-termini. Please state any need for N-terminal acetylation or C-terminal amidation during the ordering process because it is impossible to perform these modifications after synthesis.
N-terminal acetylation and C-terminal amidation reduce the overall charge of a peptide; therefore, its overall solubility might decrease. However, the stability of the peptide could also be increased because the terminal acetylation/amidation generates a closer mimic of the native protein. Therefore, these modifications might increase the biological activity of a peptide.

Advantages:
Reference: The laboratory of Michael Forma has been acetylating and amidating their blocker peptide and describing the benefits for several years. In one such publication (Biophysical Journal Volume 95 November 2008 4879–4889, Figure 2), they described the advantages of using these modifications to improve the blocking efficiency. Figure 2 in this publication provides a convincing experimental argument to suggest that the acetylating the N-terminus and amidating the C-terminus improved the channel blocking efficiency of their peptides.