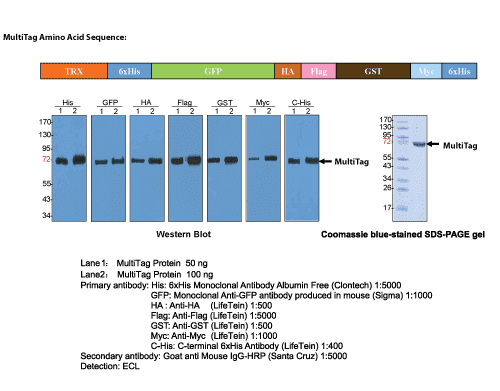
A multi-tag loading control protein is used to demonstrate that your western blotting protocol is efficient, and that the antibody recognizes target proteins that might not be present in experimental samples. Loading such positive controls results in the reliable detection of target proteins within samples. It confirms that all steps of the western blot functioned correctly, including gel electrophoresis, transfer to the membrane, membrane blocking, and antibody binding. It also gives you greater confidence that the results obtained from experimental lanes are not artifacts or non-specific binding.
We strongly recommend the use of a positive control protein when setting up new experiments to give you immediate confidence in your western blotting protocol.
When trying to detect low-abundance proteins, it is particularly important to know that your western blot is functioning as expected. If your protein of interest is detected in the control lane, then its absence in other lanes might be related to its low abundance rather than a problem with the blotting protocol.
Epitope tagging is a technique in which a known epitope is fused to a recombinant protein using genetic engineering. By choosing an epitope for which an antibody is available, it is possible to detect proteins for which no antibody is available. This is particularly useful for the characterization of newly discovered proteins and proteins of low immunogenicity.
By selecting the appropriate epitope and antibody, it is possible to select a combination with properties that are suitable for the desired experimental application, such as western blotting, immunoprecipitation, immunochemistry, or affinity purification.