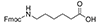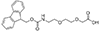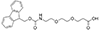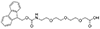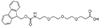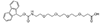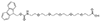Linker/Spacer
In the field of peptide synthesis, linkers play a crucial role by bridging the gap between various molecular entities, thus enabling the creation of complex peptides and proteins with desired functionalities. These linkers are not merely inert spacers; they are carefully selected to impart stability, solubility, and specificity to the resultant molecules. Among the plethora of linkers used, Fmoc-NH-PEG (Polyethylene glycol) derivatives and Aminohexanoic Acid are particularly noteworthy due to their unique properties and applications in both synthetic chemistry and biological studies.
Fmoc-NH-PEG derivatives, such as Fmoc-NH-PEG2-CH2COOH and Fmoc-NH-PEG3-CH2CH2COOH, are widely utilized in peptide synthesis for several reasons. The Fmoc (9-Fluorenylmethyloxycarbonyl) group serves as a temporary protector for amino groups, facilitating the sequential addition of amino acids in a controlled manner. The PEG (Polyethylene glycol) segment, varying in length (e.g., PEG2, PEG3), introduces solubility and flexibility into the peptide chain. This solubility is critical for otherwise insoluble peptides in aqueous or organic solvents, limiting their biological application. Moreover, the flexibility provided by the PEG linker is beneficial for peptides required to adopt specific conformations for binding to proteins or other targets in biological systems.
Aminohexanoic Acid, a simpler linker, offers a hydrophobic chain that can increase the peptide's overall hydrophobicity, influencing its interaction with biological membranes and other hydrophobic entities within the cell. This property is precious in the delivery of therapeutic peptides, where membrane permeability is a crucial factor.
The application of these linkers extends beyond mere synthesis. In biological studies, they facilitate the exploration of protein-protein interactions, enzyme-substrate relationships, and the mechanisms of action of therapeutic peptides. For instance, a peptide linked with a PEG spacer can be used to probe the active site of an enzyme without undue steric hindrance, enabling researchers to glean insights into enzyme kinetics and substrate specificity. Similarly, in protein function and structure studies, these linkers allow the attachment of fluorescent tags or other probes to peptides without significantly altering their native structure or function, thus enabling real-time tracking of peptide behavior in live cells or in vitro systems.
Furthermore, in therapeutic applications, the use of such linkers can dramatically improve the pharmacokinetic and pharmacodynamic profiles of peptide drugs. By enhancing solubility, reducing degradation by proteases, and modulating interaction with biological targets, these linkers contribute to the efficacy and safety of peptide-based therapies.
In summary, linkers like Fmoc-NH-PEG derivatives and Aminohexanoic Acid are indispensable tools in peptide synthesis and have broad implications in biological research and therapeutic development. Their ability to confer solubility, flexibility, and specific physicochemical properties to peptides opens up vast possibilities for studying and manipulating biological systems at the molecular level. As our understanding of these linkers and their interactions within complex biological matrices deepens, we can expect to see even more innovative applications in the realms of synthetic biology, drug discovery, and beyond.
| Fmoc-Glycine | 2 Carbons | 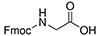 |
| 3-Amino-3-(2-Nitrophenyl) Propanoic Acid (ANP Linker) |
3 Carbons | 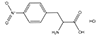 |
| Fmoc-beta-Ala-OH | 3 Carbons | 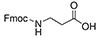 |
|
4-Aminobutyric Acid (GABA) Fmoc-GABA-OH |
4 Carbons | 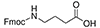 |
| 5-Aminovaleric Acid (Ava) | 5 Carbons |  |
| Aminohexanoic Acid (Ahx) | 6 Carbons |
|
|
mini-PEG or AEEA Fmoc-NH-PEG2-CH2COOH |
Length of Bonds: 9 |
|
|
mini-PEG2 or AEEP Fmoc-NH-PEG2-CH2CH2COOH |
Length of Bonds: 10 |
|
|
AEEEA Fmoc-NH-PEG3-CH2COOH |
Length of Bonds: 12 |
|
|
AEEEP, or PEG3 Fmoc-NH-PEG3-CH2CH2COOH |
Length of Bonds: 13 |
|
|
AEEEEP, PEG4 Fmoc-NH-PEG4-CH2CH2COOH |
Length of Bonds: 16 |
|
|
AEEEEEP, PEG5 Fmoc-NH-PEG5-CH2CH2COOH |
Length of Bonds: 19 |
|