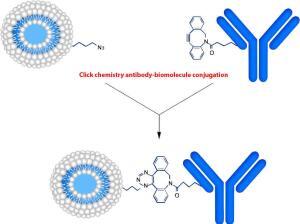
We describe a simple method for preparing antibody-peptide, antibody-oligonucleotide or antibody-compound conjugates and discuss its applications in drug delivery and new drug design. Conjugation is based on alkyne-azide cycloaddition. This Cu-free click reaction starts from the dibenzocyclooctyne (DBCO) moiety-activated antibodies and subsequently linked covalently with an azide-modified peptide, oligonucleotide or compounds. The reaction is performed under physiological conditions and has no adverse effects on antibodies or proteins. This can also be used as the click chemistry fluorescence labeling and the click chemistry in peptide-based drug design.
However, the copper-catalyzed alkyne-azide cycloaddition (CuAAC) is not suitable for applications involving functional biomolecules because copper ions can cause protein denaturation.
Measuring the protein levels directly is challenging. However, the signals can be amplified by immuno-PCR using oligonucleotide-attached antibodies to detect protein indirectly.

Antibody-Conjugate
Preparing Antibody-Peptide, Antibody Oligonucleotide or Antibody-Compound Conjugates
1. Conjugation of DBCO to the Antibody. The DBCO-PEG5-NHS was used to react with the NH2 groups on the antibody. The inclusion of a PEG5 linker improves the water solubility of the hydrophobic DBCO, introduces a spacer and flexibility between the antibody molecule and the peptide/oligonucleotide or compounds. This will alleviate the steric effect of the antibody on the enzymatic reactions.
2. Prepare the azido-Peptide or azido-oligonucleotide. LifeTein provides click chemistry modified peptide synthesis: N-terminal azide-peptide/oligo or C-terminal peptide/oligo-azide.
3. Covalent attachment of the peptide/oligonucleotide to the antibody. The reaction between DBCO and azide is slow compared to CuAAC reaction. The reaction time of 16–18 h in PBS at 4 °C is ideal to increase the final product yield. The DBCO-antibody in the intermediate reaction is stable.
https://pubs.acs.org/doi/full/10.1021/acs.bioconjchem.5b00613
Our Services:
Custom Peptide Synthesis Services
Other Posts:
Copper-Free Click Chemistry Antibody-DNA Conjugation
Personalized treatment using synthetic peptides
Long peptide synthesis by click chemistry
Post-translational modifications: Methylated peptides
Simple method to prepare antibody-peptide, antibody-oligonucleotide or antibody-compound conjugates