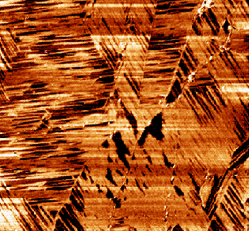
The concept of self-assembling peptides is a promising front where construction of devices can be achieved through a single molecule. While the outcome is enticing, the means to reach a consistent outcome are complex to say the least. Dozens of factors go into how a peptide may self-assemble and fold, with the most important being the sequence itself. While this can be handled by careful screening and simulations, the interface at which this folding occurs becomes more important to consider at well. Researchers looked to test how specific peptides fold and self-assemble on graphite-water interfaces, where a number of factors give this method the advantage over doing so in free solution.
Graphite helps peptides self fold into conformations
The group studying this phenomenon claimed that the folded conformations of the peptides were stable over a variety of temperatures when observed over graphite. They point out that it is due to the peptide backbone aligning with the zigzag directions of the graphite plane, thus allowing the conformations to occur more favorably from the intermolecular hydrogen bonds of the molecule. Atomic force microscopy revealed these theories to be true beyond initial simulations as well.
The team believes the design principles displayed in these experiments could be of great use in future iterations of self-assembling peptide engineering. The thermodynamically favored self-assembly with the use of a graphite-water interface shows promise as a medium for even more complex molecular devices in the future, a future LifeTein is looking forward to being a part of.
Justin Legleiter, Ravindra Thakkar, Astrid Velásquez-Silva, Ingrid Miranda-Carvajal, Susan Whitaker, John Tomich, and Jeffrey Comer
Journal of Chemical Information and Modeling 2022 62 (17), 4066-4082
DOI: 10.1021/acs.jcim.2c00419
