Cell interaction analysis is a cornerstone of biological research, providing critical insights into the intricate world of molecular communication within living organisms. While traditional microscopy offers a glimpse into these interactions, it often falls short when it comes to revealing the specific receptors and ligands involved. Enter a groundbreaking method known as Labeling Immune Partnerships by SorTagging Intercellular Contacts, or LIPSTIC for short, which has been developed by a team of innovative scientists.
At the heart of LIPSTIC lies the ingenious combination of a fluorescent LPXTG peptide motif and Staphylococcus aureus transpeptidase Sortase A (SrtA), offering a highly effective means of tracking and studying cell interactions. This novel approach is readily detectable through flow cytometry, making it a game-changer in the field of biological research.
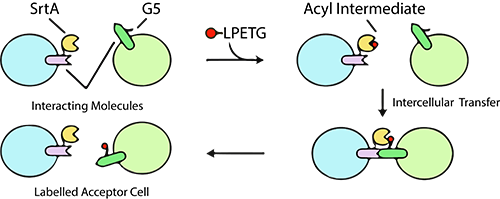
The LIPSTIC method hinges on the LPETG peptide and SrtA reaction, a technique that allows for the labeling of receptor and ligand interactions. LifeTein, a leading supplier in the life sciences industry, played a pivotal role by providing the necessary Biotin-ahx-LPETG peptide to the research group. In the LIPSTIC method, a noteworthy ligand or receptor is fused with a tag composed of five N-terminal glycine residues (G5). The SrtA enzyme then graciously donates the fluorescent peptide to this fusion, enabling precise monitoring of the acceptor cell post-separation.
One of the most impressive aspects of LIPSTIC is its versatility. It empowers scientists to analyze cell-cell interactions both in vitro and in vivo, offering a comprehensive understanding of molecular partnerships in various biological contexts. Moreover, LIPSTIC’s sensitivity is a standout feature, as it can even detect rare or low-intensity interactions that might have otherwise remained hidden.
In conclusion, the introduction of the LIPSTIC method marks a significant advancement in the field of cell interaction analysis. Its ability to unveil the intricacies of receptor-ligand interactions in living systems, along with its applicability in diverse research settings, positions LIPSTIC as a powerful tool for scientists striving to unlock the secrets of cellular communication.
Pasqual G, Chudnovskiy A, Tas JMJ, Agudelo M, Schweitzer LD, Cui A, Hacohen N, Victora GD. Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labeling. Nature. 2018 Jan 25;553(7689):496-500. doi: 10.1038/nature25442. Epub 2018 Jan 17. PMID: 29342141; PMCID: PMC5853129.