The cationic host defense peptides (CHDP), also known as antimicrobial peptides, could be used to kill enveloped viruses such as the 2019 Novel Coronavirus SARS-CoV-2. The peptides have the potential to destabilize the viral envelope on contact, damaging the virions and inhibiting infectivity. The specific antiviral peptide may bind to cellular receptors involved in viral infection or peptide-mediated aggregation of viral particles. The antiviral peptides could create an ‘antiviral shield’ at mucosal surfaces and prevent replication and spread of the Coronavirus if upregulated after the initial infection.
During pandemics, where there is insufficient time to produce vaccines (such as the outbreak of respiratory illness Covid-19 first detected in Wuhan, China), the cationic host defense peptides could be the first-line antiviral treatments.
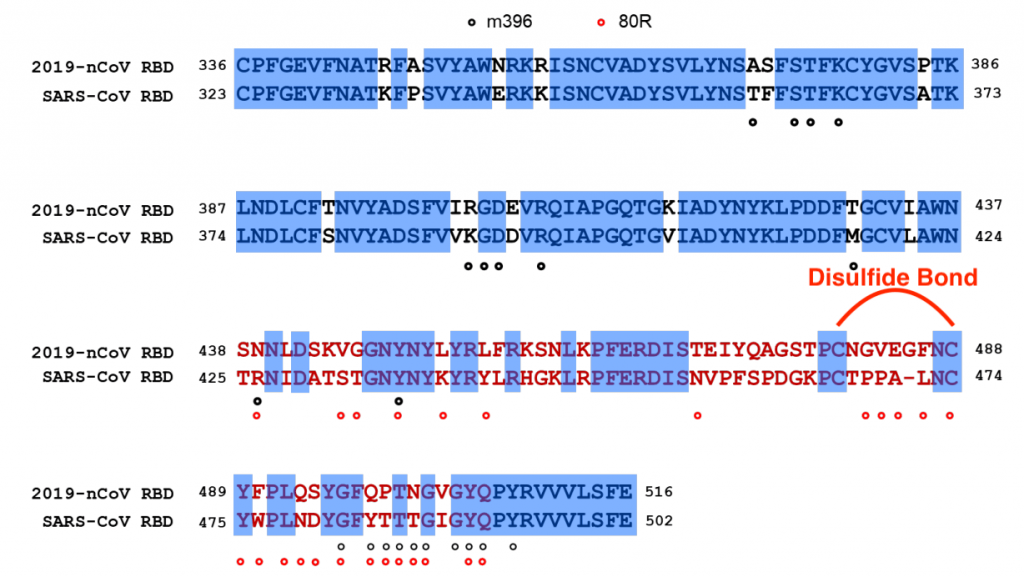
Some of the antimicrobial peptides are the human cathelicidin LL-37 and β-defensins. Cathelicidins are immunomodulatory antimicrobials with an important role in the regulation of the inflammatory response. The only human cathelicidin, LL-37, is the most well-studied peptide in this family. LL-37 is an α-helical peptide. While defensins have a common β-sheet core stabilized with three disulfide bridges between six conserved cysteine residues.
Direct antimicrobial mechanisms of cationic host defense peptides can be mediated by membrane translocation of the peptides followed by binding to intracellular targets such as nucleic acids and/or proteins to kill bacteria. Proline-rich antimicrobial peptides use inner membrane transporters as Trojan horses to gain entry and bind to intracellular targets such as nucleic acids or nascent proteins. And subsequently affect cell processes such as replication, transcription, translation, protein folding, and cell wall synthesis.
At this stage, only a few peptide-derived treatments have made it to market such as PAC-113, a histatin analog, and dalbavancin, a semisynthetic lipoglycopeptide.
Despite the limited understanding of structure-function relationships, the potential of peptide-based therapies remains a promising new clinical direction for the Coronavirus.