TMR/Cy3/Cy5 was introduced for the fluorescent label with peptides to evaluate the cell-penetrating ability and intracellular distribution of each peptide.
- Cells (HeLa or Huh-7) were seeded on 24-well culture plates (40,000 cells/well) and incubated in 400 μL of DMEM containing 10% fetal bovine serum (FBS).
- The medium was then replaced with fresh medium containing 10% FBS, and a Tetramethylrhodamine carboxylic acid (TMR)-labeled peptides
- The solution was added to each well at an appropriate concentration (for example 0.5uM, 1uM, 1.5uM, 2uM).
- After 1, 2, 3, or 4 hours of incubation, the medium was removed, and cells were washed with ice-cold PBS and trypsin.
- After the addition of medium containing 10% FBS, cells were centrifuged at 1600 rpm for 3 min at 4 °C. The cell pellets obtained were suspended in ice-cold PBS, centrifuged at 1600 rpm for 3 min at 4 °C, and then treated with Cell lysis buffer.
- The fluorescence intensity of each lysate was measured using a spectrofluorometer. The amount of protein in each well was concomitantly determined using the BCA protein assay.
- The results are presented as the mean and standard deviation obtained from 3 samples.
Reference: https://www.nature.com/articles/srep19913#s1
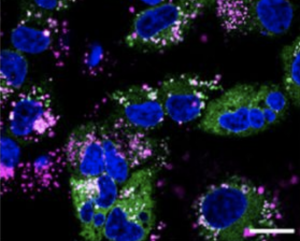
Cy5 labeled peptide in cells