Cyclic peptides as broad-spectrum antiviral agents
Antiviral drugs and vaccines are the most powerful tools to combat viral diseases. Most drugs and vaccines only target a single virus. However, the broad-spectrum antivirals can be used for rapid management of new or drug-resistant viral strains. Cyclized peptides and peptide analogs are excellent examples of broad-spectrum antivirals.
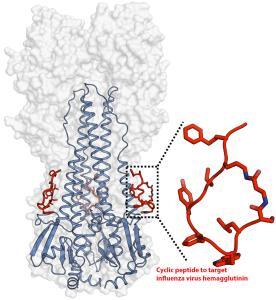
An artificial peptide molecule was found to neutralize a broad range of group 1 influenza A viruses, including H5N1. The peptide design was based on complementarity determining region (CDR) loops have been reported for other viral targets. The optimized peptides bind to the highly conserved stem epitope and block the low pH-induced conformational rearrangements associated with membrane fusion.
These peptidic compounds and their advantageous biological properties should accelerate development of novel small molecule and peptide-based therapeutics against influenza virus.
The linear peptide is Suc-SQLRSLEYFEWLSQ-NH2. Three cyclization strategies were used: head to tail, side chain to side chain and side chain to tail. An ornithine (Orn) side chain was fused with the carboxyl terminus of β-alanine for lactam formation.
Check here for more details: Potent peptidic fusion inhibitors of influenza virus, Science 28 Sep 2017, DOI: 10.1126/science.aan0516
Lately, more broad-spectrum antiviral agents were found to target viruses. It was found that 55 compounds can target eight different RNA and DNA viruses. Dalbavancin is a novel lipo-glycopeptide antibiotic. The lipoglycopeptide disrupts bacterial cell wall formation by binding to
the terminal d-alanyl-d-alanine peptidoglycan sequence in Gram-positive bacteria in a linear, concentration-dependent manner. The dalbavancin has effects on echovirus 1, ezetimibe against HIV1 and Zika virus.
More details: https://www.ncbi.nlm.nih.gov/pubmed/29698664